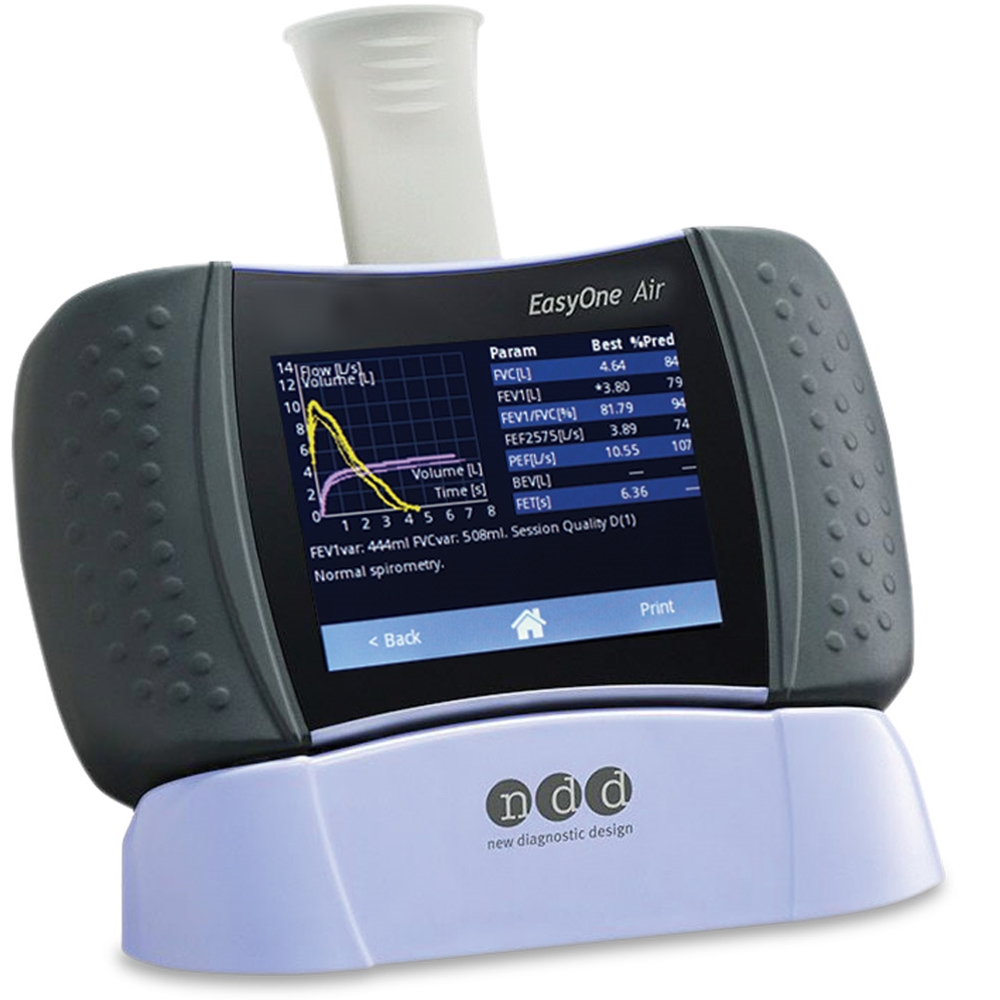
|
Technical
|
| Printing options | Direct to printer or with EasyOne Connect software |
| Data management | EasyOne Connect |
| Export | HL7, XML, GDT, with software |
| Data links | USB, Bluetooth |
| Test storage | Up to 10‘000 tests |
| Age range | Spirometry > 4 years |
| Dimensions | 87 x 155 x 36 mm (H x B x T), 356 g3.4 x 6.1 x 1.4‘‘ (H x W x D), 13 oz |
| Device classification | Type BF applied part |
| Operating conditions | Temp 0 - 40 °C/ 32 - 104 °FRel. Humidity 5 - 90%Athmosph. Pressure 700 - 1060 hPa |
| Power supply | 5 VDC, Standby 0.3W |
| Rechargeable battery | Exchangeable, 3.6 VDC |
|
Parameters
|
| FVC | BEV, EOTV, FEF10, FEF25, FEF 2575, FEF2575/FVC, FEF40, FEF50, FEF50/FVC, FEF60, FEF75, FEF80, FET, FET2575, FEV.25, FEV.5, FEV.5/FVC, FEV.75, FEV.75/FVC, FEV1, FEV1/FEV6, FEV1/FVC, FEV3, FEV3/FVC, FEV6, FVC, MEF20, MEF25, MEF40, MEF50, MEF60, MEF75, EF90, MMEF, PEF, PEFT, t0 |
| FVL | BEV, EOTV, FEF10, FEF25, FEF 2575, FEF2575/FVC, FEF40, FEF50, FEF50/FVC, FEF60, FEF75, FEF80, FET, FET2575, FEV.25, FEV.5, FEV.5/FVC, FEV.75, FEV.75/FVC, FEV1, FEV1/FEV6, FEV1/FIV1, FEV1/FVC, FEV3, FEV3/FVC, FEV6, FIF25, FIF50, FIF50/FEF50, FIF75, FIV.25, FIV.5, FIV1, FVC, MEF20, MEF25, MEF40, MEF50, MEF60, MEF75, MEF90, MIF25, MIF50, MIF75, MMEF, PEF, PEFT, t0 |
| SVC | ERV, IC, IRV, Rf, VC, VCex, VCin, VCmax, VT |
| MVV | MVV, MVV6, MVVtime, VT |
|
Predicted Normal Values
|
| GLI | Stanojevic 2009, Quanjer 2012 |
| North America | NHANES III (Hankinson) 1999, Knudson 1983, Knudson 1976, Crapo 1981, Morris 1971 & 1976, Hsu 1979, Dockery (Harvard) 1993, Polgar 1971, Gutierrez (Canada) 2004, Eigen 2001 |
| Latin America | Pereira 1992, Perreira 2006 & 2008, Pérez-Padilla (PLATINO) 2006, Pérez-Padilla (Mexico) 2001, Pérez-Padilla (Mexico, Pediatrics) 2003, Chile 2010, Chile (Pediatrics) 1997 |
| Europe | ERS (ECCS, EGKS, Quanjer) 1993, Zapletal 1977, Zapletal 2003, Rosenthal 1993, Austria 1988, Austria 1994, Sapaldia (Switzerland) 1996, Roca (Spain, SEPAR) 1982, Garcia-Rio (SEPAR) 2013, Vilozni 2005, Falaschetti 2004, Klement (Russia) 1986 |
| Europe Scandinavia | Hedenström 1985 & 1986, Gulsvik (Norway) 1985, Berglund Birath (Sweden) 1963, Langhammer (Norway) 2001, Finnish 1982 (1998), Nystad 2002 |
| Australia | Hibbert 1989, Gore Crockett 1995 |
| Asia | Chhabra (India) 2014, Dejsomritrutai (Thailand) 2000, Indonesia 1992, IP (China, HongKong) 2000 & 2006, JRS 2001 & 2014 |
| Africa | Ethiopia 1985 |
|
Flow & Volume Sensor
|
| Type | Ultrasonic transit time |
| Flow Range | ± 16 L/s |
| Flow Resolution | 4 mL/s |
| Flow Accuracy (except PEF) | ± 2% or 0.020 L/s |
| PEF Accuracy | ± 5% or 0.200 L/s |
| MVV Accuracy | ± 5% or 5 L/min |
| Volume Range | ± 12 L |
| Volume Resolution | 1 mL |
| Volume Accuracy | ± 2% or 0.050 L |
| Resistance | 0.3 cm H20/L/s at 16 L/s |
|
Standards & Recommendations
|
| Quality, Medical Devices & Electrical | ISO 13485 , ISO 14971, IEC 62366 , IEC 62304 , ISO 26782, ISO 3747 ,IEC 60601-1, IEC 60601-2, ISO 10993-1 |
| FDA | 510(k) clearance |
| Associations & Institutes | ATS/ ERS 2005, NIOSH, OSHA |
|
Languages
|
| English, French, German, Spanish |